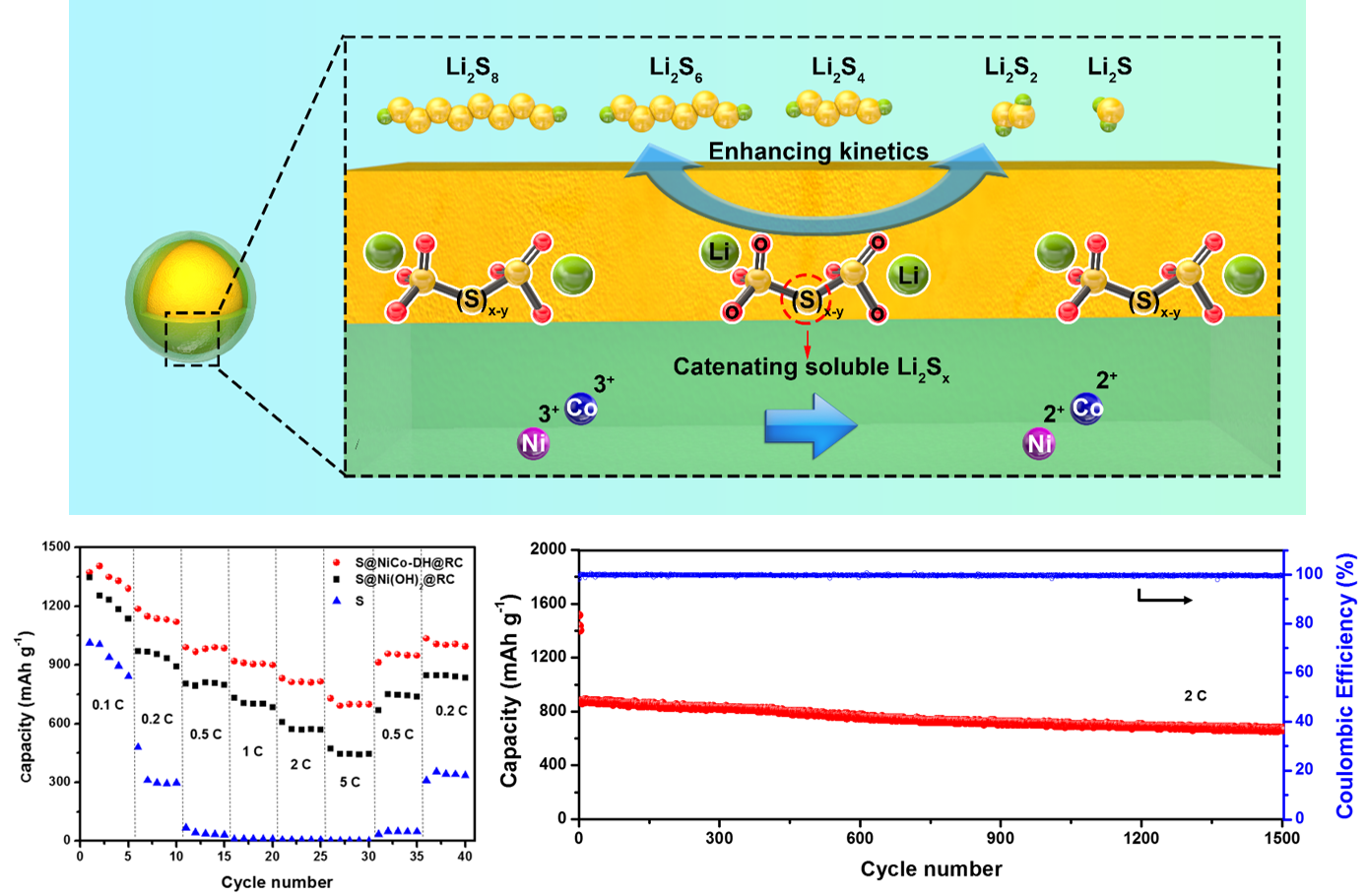
The performance of lithium–sulfur (Li–S) batteries is largely hindered by the shuttle effect caused by the dissolution of lithium polysulfides (LiPSs) and the sluggish reaction kinetics of LiPSs. Here, it is demonstrated that the nickel–cobalt double hydroxide (NiCo-DH) shells that encapsulate sulfur nanoparticles can play multiple roles in suppressing the shuttle effect and accelerating the redox kinetics of LiPSs by combining with graphene and carbon nanotubes to construct the conductive networks. The NiCo-DH shell that intimately contacts with sulfur physically confines the loss of sulfur and promotes the charge transfer and ion diffusion. More importantly, it can react with LiPSs to produce the surface-bound intermediates, which are able to anchor the soluble LiPSs and accelerate the redox kinetics. Such composite electrodes can load high contents of sulfur (>85 wt%) and the resulting Li–S battery exhibits a superior capacity (1348.1 mAh g−1at 0.1 C), ultrahigh rate performance (697.7 mAh g−1at 5 C), and ultralong cycle life (1500 cycles) with a decay rate of 0.015% per cycle.
This work has been published on Advanced Energy Materials, see details:
L. Zhang, Z. Chen, N. Dongfang, M. Li, C. Diao, Q. Wu, X. Chi, P. Jiang, Z. Zhao, L. Dong, R. Che, K. P. Loh, H. Lu, Nickel–Cobalt Double Hydroxide as a Multifunctional Mediator for Ultrahigh-Rate and Ultralong-Life Li–S Batteries. Adv. Energy Mater. 2018, DOI: 10.1002/aenm.201802431. Link